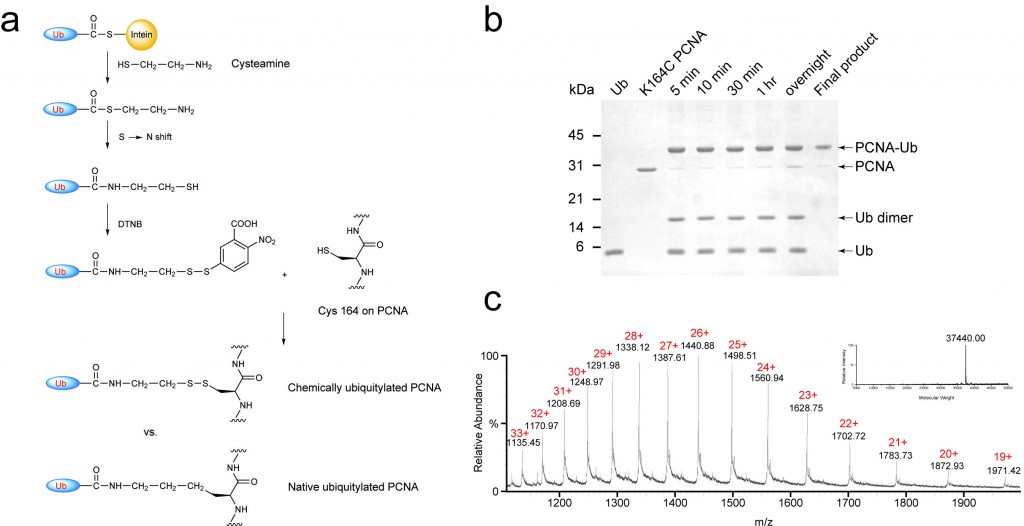
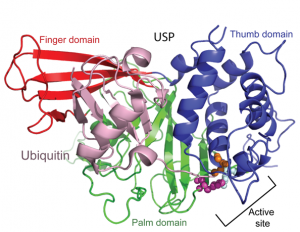
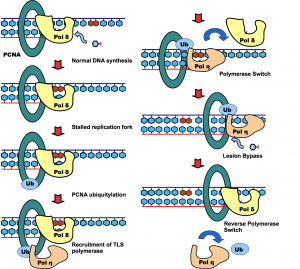
 Posttranslational modification of proteins represents a crucial way of regulating cellular functions. Modification of cellular proteins by ubiquitin and ubiquitin-like proteins plays an essential role in a number of biological processes. Ubiquitin (Ub) modification was initially discovered as a signaling mechanism for proteasome-mediated protein degradation. In recent years, ubiquitin has been found to play far broader roles in eukaryotic cells. New pathways regulated by ubiquitin are being discovered at a fast pace, virtually in almost every important aspect of cell biology, including DNA damage repair/tolerance, signal transduction, transcription, nuclear transport and innate immune response. We are investigating the eukaryotic translesion synthesis (TLS) and its regulation by ubiquitylation and SUMOylation of proliferating cell nuclear antigen (PCNA).
Posttranslational modification of proteins represents a crucial way of regulating cellular functions. Modification of cellular proteins by ubiquitin and ubiquitin-like proteins plays an essential role in a number of biological processes. Ubiquitin (Ub) modification was initially discovered as a signaling mechanism for proteasome-mediated protein degradation. In recent years, ubiquitin has been found to play far broader roles in eukaryotic cells. New pathways regulated by ubiquitin are being discovered at a fast pace, virtually in almost every important aspect of cell biology, including DNA damage repair/tolerance, signal transduction, transcription, nuclear transport and innate immune response. We are investigating the eukaryotic translesion synthesis (TLS) and its regulation by ubiquitylation and SUMOylation of proliferating cell nuclear antigen (PCNA).
We are interested in deciphering the roles of the protein or protein complexes involved in the DNA damage repair/tolerance pathways, in particular the enzymes responsible for the dynamic process of ubiquitylation or SUMOylation, as well as the functional outcomes of the post-translational modification by ubiquitin and ubiquitin-like modifier. We are also trying to discover novel ubiquitin pathways in DNA damage response using DNA microarray, bioinformatics and genetic approaches. Once we identify the target proteins we apply enzymological and structural approaches for the in-depth characterization of the protein with the goal of understanding the catalysis and regulation. Our investigations have important impact on human health, particularly, human cancer. In parallel to our mechanistic studies we are developing the small-molecule and peptidomimetic antagonists that can modulate the essential factors and enzymes in DNA damage response pathway. Some of these molecules will serve as the drug lead for the next generation of anti-cancer therapy.
Continue Reading Molecular Mechanism of DNA Translesion Synthesis