 Enzymatic ubiquitylation usually requires a number of enzymes, including ubiquitin ligase and the associated factors. The requirement of multiple enzymes limits the yield of enzymatic ubiquitylation. Chemical ubiquitylation circumvents the requirement for the ubiquitin cascade enzymes and can be readily generalized for modifying different target proteins. However, several challenges for chemical ubiquitylation also exist. Lysine residue, the site of ubiquitylation, is highly abundant in proteins. Achieving high regioselectivity is thus essential for chemical ubiquitylation. Moreover, different from other forms of post-translational modification (such as phosphorylation and methylation), ubiquitylation utilizes a small protein as modifier that itself contains many reactive groups. This represents another level of complexity for chemical ubiquitylation. We are using PCNA as a model system to develop chemical approaches for efficient protein ubiquitylation. Currently we are exploiting intein chemistry and disulfide crosslink for site-specific monoubiquitylation. The chemically ubiquitylated PCNA will be used as a probe to understand the molecular mechanism of eukaryotic translesion synthesis.
Enzymatic ubiquitylation usually requires a number of enzymes, including ubiquitin ligase and the associated factors. The requirement of multiple enzymes limits the yield of enzymatic ubiquitylation. Chemical ubiquitylation circumvents the requirement for the ubiquitin cascade enzymes and can be readily generalized for modifying different target proteins. However, several challenges for chemical ubiquitylation also exist. Lysine residue, the site of ubiquitylation, is highly abundant in proteins. Achieving high regioselectivity is thus essential for chemical ubiquitylation. Moreover, different from other forms of post-translational modification (such as phosphorylation and methylation), ubiquitylation utilizes a small protein as modifier that itself contains many reactive groups. This represents another level of complexity for chemical ubiquitylation. We are using PCNA as a model system to develop chemical approaches for efficient protein ubiquitylation. Currently we are exploiting intein chemistry and disulfide crosslink for site-specific monoubiquitylation. The chemically ubiquitylated PCNA will be used as a probe to understand the molecular mechanism of eukaryotic translesion synthesis.
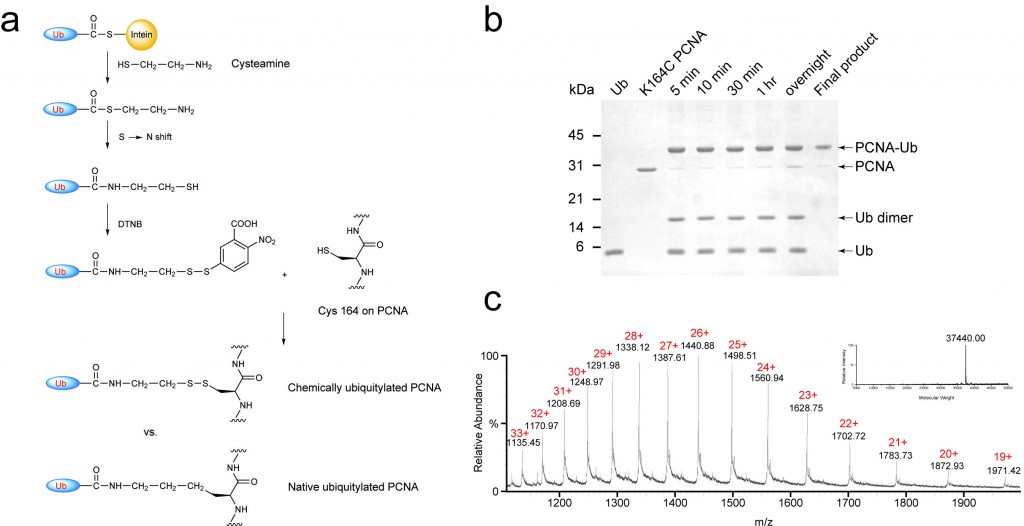
Chemical ubiquitylation of PCNA (a) The disulfide-mediated ligation between ubiquitin-cysteamine, generated by cleaving ubiquitin off chitin resin with cysteamine, and K164C PCNA. The chemically ubiquitylated PCNA mimics the native ubiquitylated PCNA. (b) Denaturing SDS-PAGE gel showing the progression of the chemical ligation reaction and the final product following an anion exchange chromatography. (c) ESI mass spectrum of the ubiquitylated PCNA. The inset shows the deconvoluted peak of K164C PCNA-Ub (37,440 Da).
Reference:
1. Yang K, Gong P, Gokhale P, Zhuang Z. Chemical Protein Polyubiquitination Reveals the Role of a Noncanonical Polyubiquitin Chain in DNA Damage Tolerance (2014) ACS Chemical Biology. ASAP, DOI:10.1021/cb500133k
2. Chen J., Ai Y., Wang J., Haracska J., Zhuang Z*. Chemically ubiquitylated PCNA as a probe for eukaryotic translesion DNA synthesis (2010) Nature Chemical Biology. 6:270.
